
For years, the power of immune-based cancer treatments has come with a perilous edge—remarkable tumor responses often shadowed by dangerous immune reactions. Now, a new contender is challenging that trade-off.
Natural killer (NK) cell therapies are yielding early clinical results that surpass expectations: robust, targeted tumor destruction without the severe cytokine storms commonly seen in some CAR-T patients. It’s a quietly radical shift that could redefine the future of safe, effective cancer immunotherapy.
Meet the Body’s Natural Cancer Killers
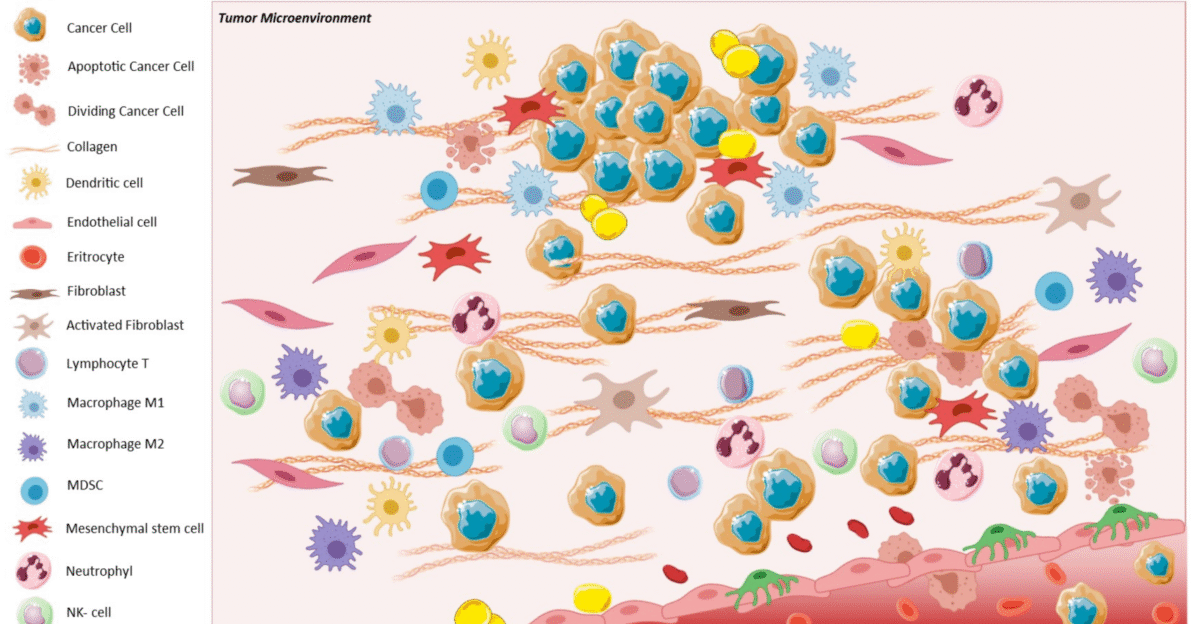
Think of NK cells as the immune system’s first responders. Unlike T cells, which must be trained to spot specific cancers, NK cells attack instantly. They don’t need personalization or genetic matching, making them easier to scale.
That simplicity has fueled an explosion of research—clinical trials jumped from just 19 in 2020 to 89 by late 2024, each exploring new ways to turn these innate defenders into precision cancer fighters.
Why CAR-T Therapy Needed a Successor
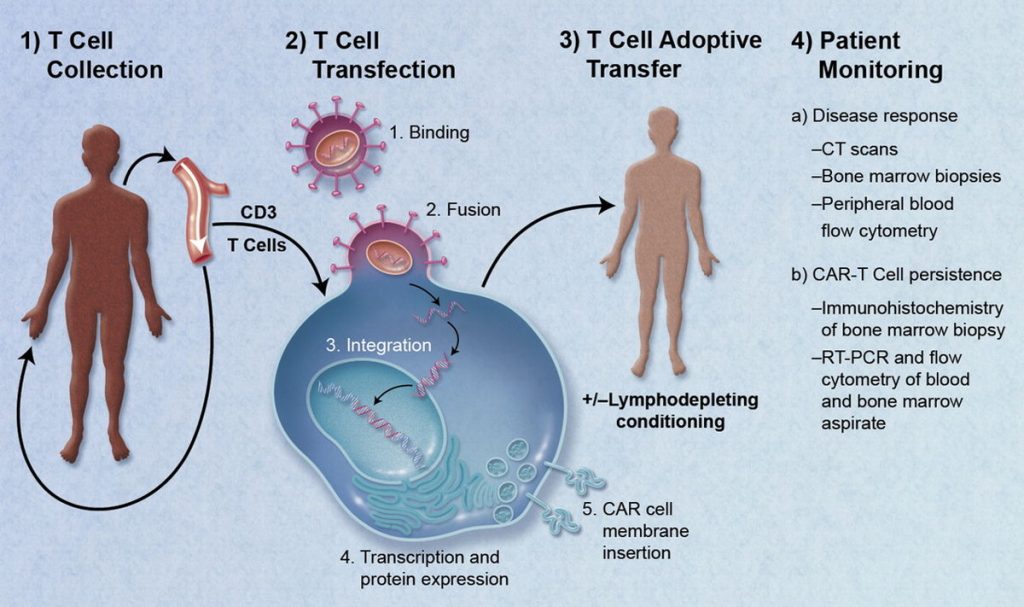
CAR-T therapy changed oncology forever, but it came with serious risks. Cytokine storms, brain inflammation, and tissue attacks can turn lifesaving treatments into dangerous ordeals. While CAR-T excels against blood cancers, its toxicity forces hospitals to monitor patients around the clock.
The need for something equally effective but far safer prompted researchers to explore NK cells—a form of immunotherapy that promises results without the life-threatening side effects.
A Safety Record That Turns Heads

When researchers combined data from 31 NK cell therapy trials, the findings stood out. Only mild side effects appeared—mostly headaches or fatigue. Not a single case of cytokine release syndrome or graft-versus-host disease occurred.
In one leukemia study, only one patient experienced a mild fever that was easily managed with steroids. For oncologists used to ICU-level monitoring after CAR-T, this safety record felt revolutionary.
The Power of ‘Off-the-Shelf’ Treatment

CAR-T therapy can take weeks and cost hundreds of thousands per patient. NK cells flip that model on its head. Because they don’t trigger immune rejection, they can be prepared in advance from healthy donors—ready to infuse when needed.
MIT’s Jianzhu Chen says this shift could bring treatment within reach for many more people, especially those with fast-moving cancers who can’t wait months for therapy to be made.
Rare Blood Cancer Sees Remarkable Turnaround
In early CD19 CAR-NK trials, two patients with Waldenstrom macroglobulinemia—a rare, treatment-resistant blood cancer—achieved complete remission. One needed only NK cells; the other combined them with rituximab. Neither experienced severe side effects.
Unlike CAR-T, both were treated entirely as outpatients. For a disease that often leaves doctors with few tools, these first responses, seen in late 2024, were a striking sign of progress.
Leukemia Trial Brings Hope and Results
For patients battling acute myeloid leukemia, NK cells offered new life. In a phase 1 trial, six of ten participants achieved complete remission within four weeks. Some remained cancer-free for more than eight months, and three went on to curative bone marrow transplants.
Side effects were minimal, mostly mild fevers that resolved on their own. Compared to traditional therapies, these outcomes mark a meaningful leap forward.
SENTI-202: Data That’s Hard to Ignore
At the 2025 AACR conference, the SENTI-202 CAR-NK trial drew headlines. Among nine patients with relapsed or refractory leukemia, nearly half achieved complete, ongoing remission. Designed with “logic-gate” technology to distinguish cancer cells from healthy ones, the treatment showed zero dose-limiting toxicities.
Lead investigator Dr. Stephen Strickland called the responses “deep and durable”, a rare phrase in trials for patients who had already run out of options.
Lung Cancer Patients See New Promise
For those with hard-to-treat lung cancer, NK therapy brought cautious optimism. In one study, 12 patients with EGFR-mutated tumors received SNK01 NK cells combined with cetuximab. Every participant saw either tumor stability or reduction—an unprecedented 100% disease control rate.
One in four had measurable tumor shrinkage, and none suffered severe side effects, even at the highest doses. For solid tumors, that’s a breakthrough in itself.
Liver Cancer Shows Extraordinary Results
In hepatocellular carcinoma, the most common liver cancer, NK therapy achieved astonishing results. Across studies, patients saw a 72% objective response rate, far surpassing existing immunotherapies.
Combining NK cells with local treatments, such as ablation or surgery, improved outcomes without increasing risk. For a cancer known for limited options, NK therapy could rewrite the standard of care.
Rewriting the Rules for Ovarian Cancer
At Dana-Farber, researchers launched a first-in-human trial using “memory-like” NK cells against platinum-resistant ovarian cancer—a devastating diagnosis with few options. These re-engineered NK cells are taught to remember and attack tumors more effectively.
Infused directly into the abdomen, early results show encouraging safety and engagement. Dr. Rebecca Porter says new strategies like this are vital as traditional immunotherapies continue to disappoint this patient group.
Boosting Power with IL-15
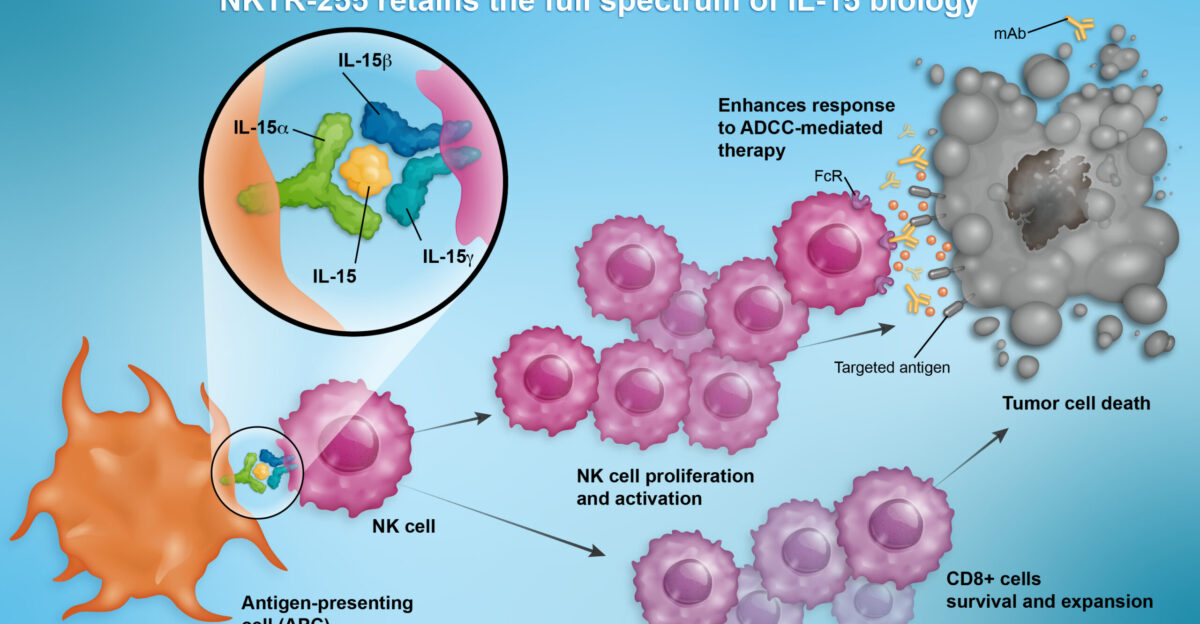
Scientists discovered that equipping NK cells with the cytokine IL-15 keeps them alive and active longer. Patients in IL-15–engineered CAR-NK trials achieved 64% complete remission rates—without the severe immune storms commonly associated with CAR-T.
Even better, the cells stayed functional for a full year. Longer persistence means fewer treatments and longer protection—turning NK therapy from a short-term fix into a sustained line of defense.
FDA Gives the Green Light

In 2024, the FDA approved ANKTIVA—an IL-15 receptor agonist that activates the body’s NK and T cells—for bladder cancer patients unresponsive to BCG. The QUILT 3.032 study showed median complete responses lasting nearly four years, with side effects in under 3% of patients.
This approval validated NK cell science in mainstream oncology and set the stage for broader use across other cancer types.
One Patient, One Miracle
Sometimes medicine changes one patient at a time. In 2025, a man with metastatic germ cell tumor—out of options—entered a trial for iNKT cell therapy. His tumors shrank by 90%, and he achieved a lasting remission.
No graft-versus-host disease, no cytokine storms—just safety and recovery. It’s a single case, but it’s proof of what NK-based therapies can achieve when everything aligns.
Smarter Design, Sharper Precision

The SENTI-202 platform uses a “logic-gate” system—like a molecular circuit—to target only leukemia cells while sparing healthy ones. Bone marrow samples confirmed it: the cancerous cells vanished, while the normal stem cells remained intact.
That precision eliminated the harsh bone marrow damage associated with chemotherapy. Dr. Strickland says this innovation “proves precision without penalty,” bringing a new standard to how targeted therapy should work.
Goodbye, Neurotoxicity

For many cancer patients, CAR-T’s scariest side effect is neurotoxicity—hallucinations, confusion, even seizures. NK therapies largely eliminate that risk. Across trials, neurotoxicity was nearly nonexistent. Scientists credit NK cells’ calmer cytokine profile, which prevents the brain inflammation that troubles CAR-T patients.
The result: people stay alert, clear-minded, and independent during treatment—a rare and welcome relief in modern oncology.
Cracking the Solid Tumor Code
Solid tumors still pose the biggest challenge. They build biochemical fortresses that block immune cells from entering. But new NK designs are closing in. IL-21–engineered NK cells, tested for glioblastoma, show better endurance and tumor infiltration than earlier IL-15 versions.
MD Anderson will test them clinically in 2025. If successful, they could finally open the door for cell therapy’s next frontier—solid tumors.
A Pipeline in Overdrive
NK cell research is booming. By late 2024, nearly 90 clinical trials were active worldwide—up from fewer than 20 just four years earlier. Studies now span lung, brain, blood, and colorectal cancers. Variants like memory-like NK cells and invariant NK-T cells are expanding rapidly.
MIT’s Jianzhu Chen predicts NK cells could someday replace CAR-T in many uses—safer, faster, and accessible to almost anyone.
Affordability Could Be the Real Revolution

Beyond safety, NK therapy could break cost barriers. CAR-T treatment costs half a million dollars and takes weeks to produce. NK therapies can be mass-manufactured in days for far less. Because they don’t require genetic matching, any patient can receive them.
For countries with limited healthcare budgets, this could be the first form of advanced cancer immunotherapy truly within reach.
A New Era of Hope Begins

Natural killer cells are changing the story of cancer therapy—from one of survival at any cost to survival with safety and dignity. Across trials, not a single severe cytokine storm or graft-versus-host case emerged.
The science now clearly indicates that immunotherapy can be both powerful and gentle. With FDA approvals and dozens of ongoing trials, NK cells aren’t just the future of cancer treatment—they’re the hope arriving now.